Corrosion
Corrosion resistance is of great significance when selecting the appropriate material for anchoring systems. The application area of metal anchoring systems in refractory constructions is essentially familiar with corrosion from chorine and chlorine compounds, sulphur and sulphur compounds as well as alkaline metals and alkaline earth metals, and more rarely from fluoride or bromide. An important influencing factor is the oxidising or reducing atmosphere present in the furnace system.
Corrosion can develop on components in the most diverse ways and to some extent is concomitant with embrittlement processes. Corrosion is understood as the reaction of metals to surrounding substances that is accompanied by a (negative) change to the original properties of the material. The direct reaction of the metal with a reaction partner is described as chemical corrosion.
This almost always involves oxidation, which is also known as scaling in high temperature corrosions. Electrochemical corrosion refers to stress resulting from the reaction of the material generally to aqueous media, also where originally gaseous media have dropped below the dew point, or to other metals.

Surface corrosion is evident by virtue of material erosion, as extensive corrosion or pitting, as well as in the form of single or multiple surface layer formation, which can frequently be accompanied by internal corrosion under the surface layer.
The formation of surface layers can be accompanied by a great increase in volume, leading to flaking. In addition, considerable stress can arise in the refractory lining which leads to failure of the refractory material. The surface corrosion in anchoring systems reduces the retention cross-section of the component and gradually leads to failure of the anchor. The result is that the refractory lining falls out.
Internal corrosion attacks the lattice structure of the metal and has a negative effect on the structure of the component and on creep strength. Individual alloy components can be attacked which are then no longer available for corrosion resistance or there may also be intercrystalline corrosion, in which changes take place to the grain boundaries of the metal matrix due to the precipitation of chromium-enriched phases.
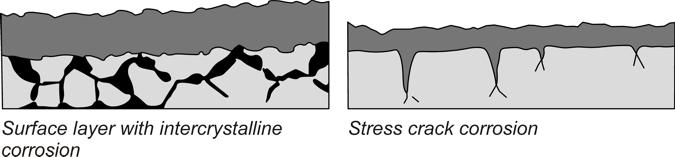
The stress corrosion usually takes place without outer signs and can occur as intercrystalline or transcrystalline stress corrosion. In any event, a mechanical influence, such as tensile stress, must be present in addition to the corrosive medium.
The corrosion attack from sulphur and sulphur compounds can take place in different ways. In the case of gaseous sulphur compounds it is a gas-solids reaction that creates a compound of the sulphur and the alloy components of the metal. In the case of falling below the dew point, aqueous solutions of sulphuric acids develop that cause corrosion or pitting corrosion. Molten salts can develop in conjunction with alkaline metals and alkaline earth metals which likewise attack and weaken the metal matrix.

The corrosion in gaseous chlorine compounds in a gas-solids reaction occurs in the same way as with the sulphur compounds. The hydrochloric acids that develop when falling below the dew point can lead to pitted corrosion or, where mechanical stress is present at the same time, to stress corrosion. Where chlorine molten salts develop, the metal eroded from the surface.
Aqueous leach solutions may be created where there is a drop below the dew point in conjunction with alkaline metals and alkaline earth metals; these can erode the metal and lead to stress corrosion in conjunction with mechanical stress.
